
Atoms Around Us
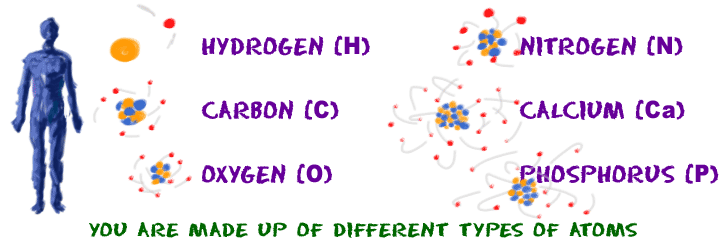
What is an atom? Atoms are building blocks. If you want to create a language, you'll need an alphabet. If you want to build molecules, you will need atoms from different elements. Elements are the alphabet in the language of molecules.
Each element is a little bit different from the rest. In English, you know that “B” is different from “C.” In chemistry, “B” is also different from “C” because boron (B) and carbon (C) are different elements with atoms that have different structures. Atoms are defined as the smallest units of matter that have the properties of an individual element.
Are there pieces of matter smaller than an atom? Sure. Atoms are made up of smaller and even smaller particles of matter. However, those smaller particles don’t have the properties of an element. The electrons in a gold (Au) atom are the same as the electrons in an atom of neon (Ne). In chemistry, we like to study atoms because the Universe revolves around the properties of elements, not necessarily the properties of an electron or proton.
For example, let’s say you have bar of gold. You know that bar is made of matter because everything is made of matter. Because it is pure gold, there are only gold atoms in the bar. If you only had one atom of gold in your hand, it would have the same properties as every other gold atom in that bar. But what if you only had one electron from a gold atom? That electron would not have the properties of gold anymore. It would just be an electron doing electron stuff. The atom is the smallest unit that has the properties of an element.
We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work.
Common Elements
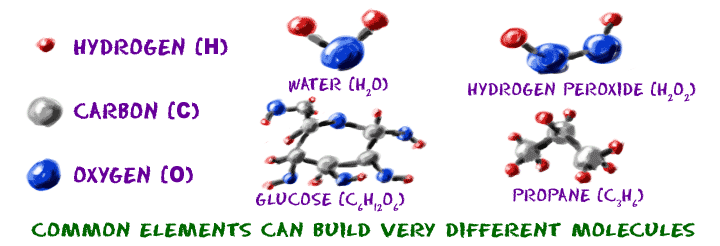
Let's work with the alphabet idea again. If you read a book, you will find words on each page. Letters make up those words. In English, we only have twenty-six letters, but we can make thousands of words. In chemistry, you are working with almost 120 elements. When you combine them, you can make millions of different molecules.
Molecules are groups of atoms in the same way that words are groups of letters. An "A" will always be an "A" no matter what word it is in. A sodium (Na) atom will always be a sodium atom no matter what molecule it is in. While atoms from different elements have different masses and structures, they are all built with the same parts. Electrons, protons, and neutrons are the basic subunits for all atoms across the Universe.
From Simple to Complex
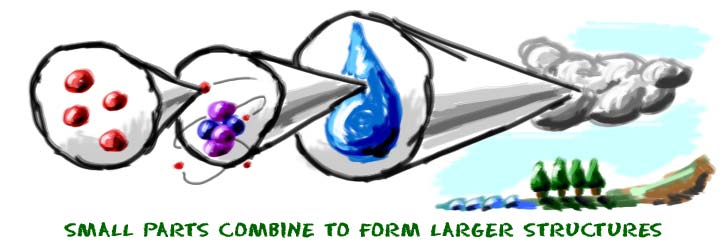
If you want to do a little more thinking, imagine the smallest particles of matter. Super-tiny subatomic particles are used to create the parts of atoms. Protons, neutrons, and electrons can then organize to form atoms. Atoms are then used to create the molecules around us. As we just learned, there are almost 120 elements that can be found in the molecules we know. Smaller molecules can work together and build macromolecules. It just goes on. Everything you see or imagine is built from something else.
You could start really small...
- Particles of matter
- Atoms
- Molecules
- Macromolecules
- Cell organelles
- Cells
- Tissues
- Organs
- Systems
- Organisms
- Populations
- Ecosystems
- Biomes
- Planets
- Systems with Stars
- Galaxies
- The Universe
...And finish really big.
Wow! All of that is possible because of atoms.
► NEXT PAGE ON ATOMS
► NEXT STOP ON SITE TOUR
► ATOMS QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► NEXT STOP ON SITE TOUR
► ATOMS QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
A Star Turns Inside Out (NASA Video)
Encyclopædia Britannica: Atoms
Wikipedia: Atoms
Wikipedia: Atomic Clock
Wikipedia: Particle Accelrators
Encyclopedia.com: Matter
Thomas Jefferson National Labs: Atom Tour
NYU.edu: What are Atoms?



