
Dangerous Particles
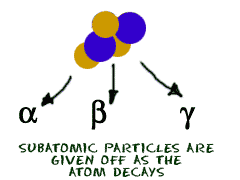 Radioactivity occurs when an atomic nucleus breaks down into smaller particles. There are three types of particles: alpha, beta, and gamma. Alpha particles are positively charged, beta particles are negatively charged, and gamma particles have no charge. The particles also have increasing levels of energy. Alpha has the lowest energy, beta has a bit more, and then gamma is the fastest and most energetic of all the emission particles.
Radioactivity occurs when an atomic nucleus breaks down into smaller particles. There are three types of particles: alpha, beta, and gamma. Alpha particles are positively charged, beta particles are negatively charged, and gamma particles have no charge. The particles also have increasing levels of energy. Alpha has the lowest energy, beta has a bit more, and then gamma is the fastest and most energetic of all the emission particles.
The term half-life describes the time it takes for the amount of radioactivity to go down by one-half. Let's say you have some uranium (U) (don't try this at home!) and it's radioactive. When your measurements tell you that the level of radioactivity has gone down by one-half, the amount of time that has passed is the half-life. Every isotope has its own unique half-life. The half-life of uranium-235 is 713,000,000 years. The half-life of uranium-238 is 4,500,000,000 years. That's a long time to wait for the radioactivity to decrease.
Harnessing the Energy
Nuclear energy is the energy released when the nuclei (nuclei is the plural of nucleus) of atoms split or are fused. You know the nucleus is made up of protons and neutrons. Nuclear forces hold all of the pieces together. Fusion is when two nuclei come together. Fission is when one nucleus is split into two or more parts. Huge amounts of energy are released when either of these reactions occurs. Fusion reactions create much of the energy given off by the Sun. Fission creates the much smaller particles that make up the protons and neutrons that physicists are studying every day. In our nuclear reactors, fission is the main process. In the Sun, fusion is the big process.Atoms from the Mirror Universe
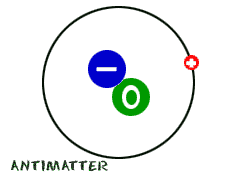 Since we're talking a little bit about atomic and nuclear physics, we wanted to tell you about antimatter. It's not just found in television shows. Scientists have proved that it is real. While a regular atom has positive and neutral pieces (protons/neutrons) in the nucleus and negative pieces in orbiting clouds (electrons), antimatter is just the opposite. Antimatter has a nucleus with a negative charge and little positive pieces in the orbits. Those positively charged pieces are called positrons. According to news reports in 2010, scientists at CERN (a particle collider) created antihydrogen atoms. They couldn't really do anything with them, since they lasted for less than a second... but they made them!
Since we're talking a little bit about atomic and nuclear physics, we wanted to tell you about antimatter. It's not just found in television shows. Scientists have proved that it is real. While a regular atom has positive and neutral pieces (protons/neutrons) in the nucleus and negative pieces in orbiting clouds (electrons), antimatter is just the opposite. Antimatter has a nucleus with a negative charge and little positive pieces in the orbits. Those positively charged pieces are called positrons. According to news reports in 2010, scientists at CERN (a particle collider) created antihydrogen atoms. They couldn't really do anything with them, since they lasted for less than a second... but they made them!
Related Video...
Dark Lightning (Science@NASA Video)
Encyclopædia Britannica: Radioactive Isotopes
Wikipedia: Radioactive Decay
Encyclopedia.com: Antimatter



