
Neither Here nor There
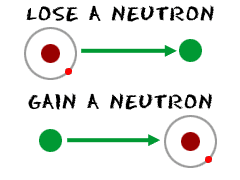 Neutrons are the particles in an atom that have a neutral charge. They aren't positive like protons. They aren't negative like electrons. But don't start thinking that they aren't important. Every piece of an atom has huge importance to the way the atom acts and behaves. Neutrons are no exception.
Neutrons are the particles in an atom that have a neutral charge. They aren't positive like protons. They aren't negative like electrons. But don't start thinking that they aren't important. Every piece of an atom has huge importance to the way the atom acts and behaves. Neutrons are no exception.
So, if an atom has equal numbers of electrons and protons, the charges cancel each other out and the atom has a neutral charge. You could add a thousand neutrons into the mix and the charge would not change. However, if you add a thousand neutrons, you will be creating one super-radioactive atom. Neutrons play a major role in the mass and radioactive properties of atoms. You may have read the page on isotopes. Isotopes are created when you change the normal number of neutrons in an atom.
Inside the Nucleus
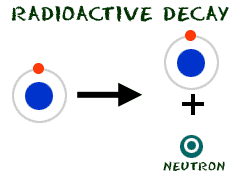 You know that neutrons are found in the nucleus of an atom. Under normal conditions, protons and neutrons stick together in the nucleus. During radioactive decay, they may be knocked out of there. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. If there are many atoms of an element that are isotopes, the average atomic mass for that element will change. We have spoken about carbon (C) having an average mass of 12.01. It's not much different than you would expect from an atom with 6 protons and 6 neutrons. The number of carbon isotopes doesn't change the atomic mass very much. As you move higher in the periodic table, you will find elements with many more isotopes.
You know that neutrons are found in the nucleus of an atom. Under normal conditions, protons and neutrons stick together in the nucleus. During radioactive decay, they may be knocked out of there. Neutron numbers are able to change the mass of atoms, because they weigh about as much as a proton and electron together. If there are many atoms of an element that are isotopes, the average atomic mass for that element will change. We have spoken about carbon (C) having an average mass of 12.01. It's not much different than you would expect from an atom with 6 protons and 6 neutrons. The number of carbon isotopes doesn't change the atomic mass very much. As you move higher in the periodic table, you will find elements with many more isotopes.
One Special Element
Did we say that all atoms have neutrons? Oops. All elements have atoms with neutrons except for one. A normal hydrogen (H) atom does not have any neutrons in its tiny nucleus. That tiny little atom (the tiniest of all) has only one electron and one proton. You can take away the electron and make an ion, but you can't take away any neutrons. Hydrogen's special structure becomes very important when you learn how hydrogen interacts with other elements in the periodic table. If you learn about nuclear fusion you will learn about deuterium and tritium. Deuterium is a hydrogen atom with an extra neutron and tritium has two extra. You won't find much deuterium in your backyard. It's mainly in oceans. Don't worry if you do find it, it's not radioactive. It's a stable isotope.Related Video...
Superfluid Cores of Stars (Science@NASA Video)



