
Liquid to a Gas and Back to a Liquid
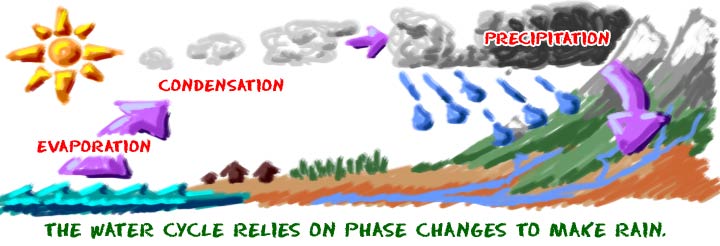
When you are a liquid and want to become a gas, you need to find a lot of energy. Once you can direct that energy into your molecules, they will start to vibrate. If they vibrate enough, they can escape the limitations of the liquid environment and become a gas. When you reach your boiling point, the molecules in your system have enough energy to become a gas.
The reverse is true if you are a gas. You need to lose some energy from your very excited gas atoms. The easy answer is to lower the surrounding temperature. When the temperature drops, energy will be transferred out of your gas atoms into the colder environment. When you reach the temperature of the condensation point, you become a liquid. If you were water vapor over a boiling pot of water and you hit a wall, the wall would be cool, absorb some of your extra energy, and you could quickly become a liquid. Cooler objects often absorb energy from hotter objects.
Gas to a Plasma and Back to a Gas
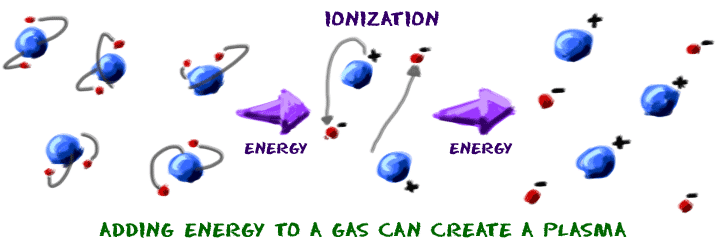
Let's finish up by imagining you're a gas like neon (Ne). You say, "Hmmmm. I'd like to become a plasma. They are too cool!" As a gas, you're already halfway there, but you still need to tear off a bunch of electrons from your atoms. The gas needs to ionize. Electrons have a negative charge. Eventually, you'll have groups of positively and negatively charged particles in almost equal concentrations. They wind up in a big plasma ball. Because the positive and negative charges are in equal amounts, the charge of the entire plasma is close to neutral. Neutral happens when a whole bunch of positive particles cancel out the charges of an equal bunch of negatively charged particles.
Plasma can be made from a gas if a lot of energy is pushed into the gas. In the case of neon, it is electrical energy that pulls the electrons off. When it is time to become a gas again, just flip the neon light switch off. Without the electricity to energize the atoms, the neon plasma returns to its gaseous state. We have a special world here on Earth. We have an environment where you don't find a lot of everyday plasma. Once you leave Earth and travel through the Universe, you will find plasma everywhere. It's in the stars and all of the space in between.
More on Phase Changes from Part I...
Examples of Phase Changes
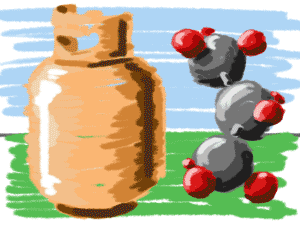
Gases Under Pressure
If you have a propane (C3H8) barbecue, you have probably seen those cylinders filled with fuel. In the cylinder, the propane molecules are in a liquid state at a high pressure. When the molecules are released from the cylinder, they immediately become a gas and you can cook your food. Pressure differences make the phase change.
Melting Ice
You can watch phase changes at home when you put a piece of ice (solid) on a counter. As long as the temperature is above 0 degrees Celsius, that ice cube will warm and melt. That melted puddle of water (H2O) is a liquid. Heat makes the phase change. Have a towel ready to clean up the mess.
Smelling Steak
Let’s say you’re cooking when you go camping or at a barbecue at home. When you put the food on the grill, you don’t smell much. As the food heats up, aromatic molecules begin to escape the surface of the food and diffuse through the air. Those volatile molecules are heated up and become a gas when they evaporate. Heat makes the phase change.
Plasma in Tubes
In many signs, there are glass tubes filled with neon (Ne) gas. Normally, the gas stays a gas. When you turn on the sign and send an electric current through the tubes, the neon loses its electrons and becomes plasma. Electricity makes the phase change.Related Video...
The Mystery of the Aurora (NASA Video)
Encyclopædia Britannica: Phase Transitions
Wikipedia: Phase Changes
Wikipedia: Phase Transitions
Encyclopedia.com: States of Matter
University of Colorado: States of Matter
UC Davis: Phase Trasitions



