
Solid Basics
What is one physical characteristic of a solid? Solids can be hard like a rock, soft like fur, a big rock like an asteroid, or small rocks like grains of sand. The key is that solids hold their shape and they don't flow like a liquid. A rock will always look like a rock unless something happens to it. The same goes for a diamond. Solids can hold their shape because their molecules are tightly packed together.
You might ask, "Is baby power a solid? It's soft and powdery." Baby power is also a solid. It's just a ground down piece of talc. Even when you grind a solid into powder, you will see tiny pieces of that solid under a microscope. Liquids will flow and fill up any shape of container. Solids like to hold their shape.
 In the same way that a large solid holds its shape, the atoms inside of a solid are not allowed to move around too much. Atoms and molecules in liquids and gases are bouncing and floating around, free to move where they want. The molecules in a solid are stuck in a specific structure or arrangement of atoms. The atoms still vibrate and the electrons fly around in their orbitals, but the entire atom will not change its position.
In the same way that a large solid holds its shape, the atoms inside of a solid are not allowed to move around too much. Atoms and molecules in liquids and gases are bouncing and floating around, free to move where they want. The molecules in a solid are stuck in a specific structure or arrangement of atoms. The atoms still vibrate and the electrons fly around in their orbitals, but the entire atom will not change its position.
Solid Mixtures
Solids can be made of many things. They can have pure elements or a variety of compounds inside. When you have a solid with more than one type of compound, it is called a mixture. Most rocks are mixtures of many different compounds. Concrete is a good example of a man-made solid mixture.Granite is a mixture you might find when you hike around a national park. Granite is made of little pieces of quartz, mica, and other particles. Because all of the little pieces are spread through the rock in an uneven way, scientists call it a heterogeneous mixture. Heterogeneous mixtures have different concentrations of compounds in different areas of the mixture. For example, there might be a lot of quartz and very little feldspar in one part of the granite, but only a few inches away those amounts might flip.
Crystals
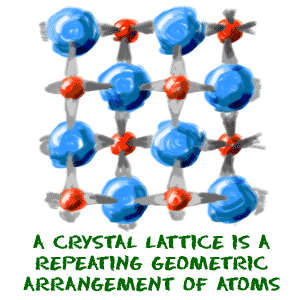 On the other end of the spectrum is something called a crystal. A crystal is a form of solid where the atoms are arranged is a very specific order. Crystals are often pure substances and not all substances can form crystals because it is a very delicate process. The atoms are arranged in a regular repeating pattern called a crystal lattice. Table salt (NaCl) is a great example of a crystal you can find around your house. The sodium (Na) and chlorine (Cl) atoms arrange themselves in a specific pattern to form the cubic salt crystals.
On the other end of the spectrum is something called a crystal. A crystal is a form of solid where the atoms are arranged is a very specific order. Crystals are often pure substances and not all substances can form crystals because it is a very delicate process. The atoms are arranged in a regular repeating pattern called a crystal lattice. Table salt (NaCl) is a great example of a crystal you can find around your house. The sodium (Na) and chlorine (Cl) atoms arrange themselves in a specific pattern to form the cubic salt crystals.
Allotropes
A diamond is another good example of a crystal. Diamonds are a crystal form of pure carbon (C). The carbon atoms of a diamond are connected in a very compact and structured way. The carbon atoms found in graphite (in pencils) have a different crystalline arrangement. According to the Mohs hardness scale, diamonds are very hard with a value of 10 while graphite is very soft with a value of 1.5. The two different structures of carbon atoms (tetrahedron versus hexagon) are called allotropes.
► NEXT PAGE ON MATTER
► NEXT STOP ON SITE TOUR
► SOLIDS QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► NEXT STOP ON SITE TOUR
► SOLIDS QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
Get Inspired Outdoors - Youth Programs (US Nat'l Parks Video)
Encyclopædia Britannica: Solids
Wikipedia: Solids
Encyclopedia.com: Solids
Caltech.edu: Solids
Eastern New Mexico University: Diamond and Graphite
BBC.co.uk: Solids and Liquids



