
Acids and Bases Are Everywhere
Every liquid you see will probably have either acidic or basic traits. Water (H2O) can be both an acid and a base, depending on how you look at it. It can be considered an acid in some reactions and a base in others. Water can even react with itself to form acids and bases. It happens in really small amounts, so it won't change your experiments at all. It goes like this:2H2O --> H2O + H+ + OH- --> H3O+ + OH-
See how the hydrogen ion was transferred?
Most of the time, the positive and negative ions in distilled water are in equal amounts and cancel each other out. Most water you drink from the tap has other ions in it. Those special ions in solution make something acidic or basic. In your body there are small compounds called amino acids. The name tells you those are acids. In fruits there is something called citric acid. That's an acid too. But what about baking soda? When you put that in water, it creates a basic solution. Vinegar? Acid.
So what makes an acid or a base? A chemist named Svante Arrhenius came up with a way to define acids and bases in 1887. He saw that when you put molecules into water, sometimes they break down and release an H+ (hydrogen) ion. At other times, you find the release of an OH- (hydroxide) ion. When a hydrogen ion is released, the solution becomes acidic. When a hydroxide ion is released, the solution becomes basic. Those two special ions determine whether you are looking at an acid or a base. For example, vinegar is also called acetic acid. (Okay, that gives away the answer.) If you look at its atoms when it's in water, you will see the molecule CH3COOH split into CH3COO- and H+. That hydrogen ion is the reason it is called an acid. Chemists use the word "dissociated" to describe the breakup of a compound.
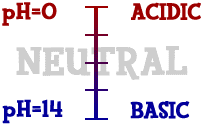 Scientists use something called the pH scale to measure how acidic or basic a liquid is. Although there may be many types of ions in a solution, pH focuses on concentrations of hydrogen ions (H+) and hydroxide ions (OH-). The scale measures values from 0 all the way up to 14. Distilled water is 7 (right in the middle). Acids are found between 0 and 7. Bases are from 7 to 14. Most of the liquids you find every day have a pH near 7. They are either a little below or a little above that mark. When you start looking at the pH of chemicals, the numbers can go to the extremes. If you ever go into a chemistry lab, you could find solutions with a pH of 1 and others with a pH of 14. There are also very strong acids with pH values below 1, such as battery acid. Bases with pH values near 14 include drain cleaner and sodium hydroxide (NaOH). Those chemicals are very dangerous.
Scientists use something called the pH scale to measure how acidic or basic a liquid is. Although there may be many types of ions in a solution, pH focuses on concentrations of hydrogen ions (H+) and hydroxide ions (OH-). The scale measures values from 0 all the way up to 14. Distilled water is 7 (right in the middle). Acids are found between 0 and 7. Bases are from 7 to 14. Most of the liquids you find every day have a pH near 7. They are either a little below or a little above that mark. When you start looking at the pH of chemicals, the numbers can go to the extremes. If you ever go into a chemistry lab, you could find solutions with a pH of 1 and others with a pH of 14. There are also very strong acids with pH values below 1, such as battery acid. Bases with pH values near 14 include drain cleaner and sodium hydroxide (NaOH). Those chemicals are very dangerous.
More information in part two.
► NEXT PAGE ON CHEMICAL REACTIONS
► ACIDS AND BASES QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► ACIDS AND BASES QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
Ocean Acidification in the Arctic (USGS Video)



