
Equilibrium Basics
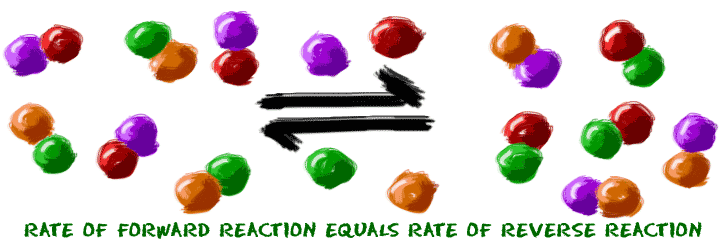
Equilibrium is a pretty easy topic - big name, but easy idea. First, when you have a system made up of a bunch of molecules, those molecules sometimes combine. That's the idea of a chemical reaction. Second, a chemical reaction sometimes starts at one point and moves to another. Now imagine the reaction finished and you have a pile of new chemicals. Guess what? Some of those chemicals want to go through a reverse chemical reaction and become the original molecules again. We don't know why. Sometimes they just do.
Put those two ideas together and you have equilibrium:
1. Two reactants combine to make a product.
2. Products like to break apart and turn back into the reactants.
 There is a point where those two reactions happen and you can't tell that any reactions are happening. That's the point when the reaction looks like it is finished. In reality, some of the molecules are turning into products and some are turning back into reactants. You need to imagine that you're as small as a molecule and you're watching all of these parts bouncing around and changing back and forth. Just staring at a test tube, you won't generally notice a change in their numbers. That's what equilibrium really is. The overall reaction is happy. There is no pressure greater in one direction over another.
There is a point where those two reactions happen and you can't tell that any reactions are happening. That's the point when the reaction looks like it is finished. In reality, some of the molecules are turning into products and some are turning back into reactants. You need to imagine that you're as small as a molecule and you're watching all of these parts bouncing around and changing back and forth. Just staring at a test tube, you won't generally notice a change in their numbers. That's what equilibrium really is. The overall reaction is happy. There is no pressure greater in one direction over another.
There are some other traits of equilibrium. Equilibrium always happens at the same point in the reaction no matter where you start. So, if you start with all of substance A, it will break up and become B and C. Eventually, B and C will start recombining to become A. Those reactions happen until they reach equilibrium. They reach equilibrium at the same point whether you start with all A, all B/C, or half A and half B/C. It doesn't matter. There is one special point where the forward and reverse reactions cancel each other out.
It Happens on Its Own
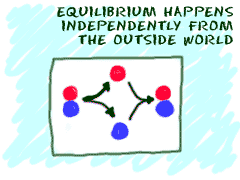 Another idea is that equilibrium is reached by itself with no outside forces acting on the system. If you put two substances in a mixture, they can combine and react by themselves. Eventually, they will reach equilibrium. Scientists say that equilibrium happens through spontaneous processes. They happen on their own.
Another idea is that equilibrium is reached by itself with no outside forces acting on the system. If you put two substances in a mixture, they can combine and react by themselves. Eventually, they will reach equilibrium. Scientists say that equilibrium happens through spontaneous processes. They happen on their own.
There is one last idea. Do you remember that some atoms and molecules have charges? A system "at equilibrium" appears to have no charge (neutral). All the pluses and minuses cancel each other out and give a total charge of "0". Scientists use the letter "K" to add up all of the actions and conditions in a reaction. That "K" is the equilibrium constant.
Learn more about equilibrium in part two.
► NEXT PAGE ON CHEMICAL REACTIONS
► NEXT STOP ON SITE TOUR
► EQUILIBRUM QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► NEXT STOP ON SITE TOUR
► EQUILIBRUM QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
Measuring Criegee Intermediate Reactions (Sandia Nat’l Labs Video)
Encyclopædia Britannica: Chemical Equilibrium
Wikipedia: Chemical Equilibrium
Encyclopedia.com: Equilibrium



