
Names to Know
Let's look at the whole picture now. There is a scale for acids and bases just like everything else. Here are a couple of definitions you should know:Acid: A solution that has an excess of H+ ions. It comes from the Latin word acidus, which means "sharp" or "sour".
Base: A solution that has an excess of OH- ions. Another word for base is alkali.
Aqueous: A solution that is mainly water. Think about the word aquarium. AQUA means water.
Strong Acid: An acid that has a very low pH (0-4).
Strong Base: A base that has a very high pH (10-14).
Weak Acid: An acid that only partially ionizes in an aqueous solution. This means that not every molecule breaks apart. Weak acids usually have a pH close to 7 (3-6).
Weak Base: A base that only partially ionizes in an aqueous solution. This means that not every molecule breaks apart. Weak bases usually have a pH close to 7 (8-10).
Neutral: A solution that has a pH of 7. It is neither acidic nor basic.
More Ideas About Acids and Bases
We told you about that guy Arrhenius and his ideas about concentrations of hydrogen and hydroxide ions. You're also going to learn about Brønsted-Lowry ideas. These two chemists from Denmark and England looked at acids as donors and bases as acceptors. What were they donating and accepting? Hydrogen ions. It's a lot like the first definition we gave, where an acid breaks up and releases/donates a hydrogen ion. This newer definition is a little bit more detailed. Scientists used the new definition to describe more bases, such as ammonia (NH3). Since bases are proton acceptors, when ammonia was seen accepting an H+ and creating an ammonium ion (NH4+), it could be labeled as a base. You didn't have to worry about hydroxide ions anymore. If it got the H+ from a water molecule, then the water (H2O) was the proton donor. Does that mean the water was the acid in this situation? Yes.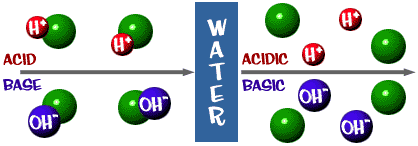
A chemist named Lewis offered a third way to look at acids and bases. Instead of looking at hydrogen ions, he looked at pairs of electrons (remember our pictures with dot structures in Atoms and Elements?). In Lewis' view, acids accept pairs of electrons and bases donate pairs of electrons. We know that both of these descriptions of acids and bases use completely opposite terms, but the idea is the same. Hydrogen ions still want to accept two electrons to form a bond. Bases want to give them up. Overall, Lewis' definition was able to classify even more compounds as acids or bases.
What Really Happens?
What really happens in those solutions? It gets a little tricky here. Let's look at the breakup of molecules in aqueous (water-based) solutions one more time for good measure. Acids are compounds that dissociate (break) into hydrogen (H+) ions and another compound when placed in an aqueous solution. Remember that acetic acid example? Bases are compounds that break up into hydroxide (OH-) ions and another compound when placed in an aqueous solution. We'll talk about baking soda in a few paragraphs.Let's change the wording a bit. If you have an ionic/electrovalent compound and you put it in water, it will break apart into two ions. If one of those ions is H+, the solution is acidic. The strong acid hydrogen chloride (HCl) is one example. If one of the ions is OH-, the solution is basic. An example of a strong base is sodium hydroxide (NaOH). There are other ions that make acidic and basic solutions, but we won't be talking about them here.
That pH scale we talked about is actually a measure of the number of H+ ions in a solution. If there are a lot of H+ ions, the pH is very low. If there are a lot of OH- ions compared to the number of H+ ions, the pH is high.
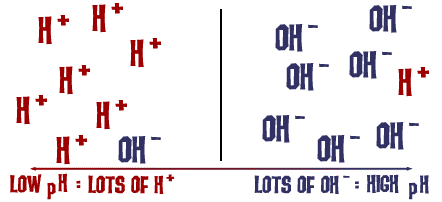
That's basically it. (Ha ha! Get it?)
More information in part one.
► NEXT PAGE ON CHEMICAL REACTIONS
► ACIDS AND BASES QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
► ACIDS AND BASES QUIZ
► RETURN TO TOP OF PAGE
► Or search the sites...
Related Video...
Starting Fire in Water (Science@NASA Video)



